

When the liquid on the left is back at the reference mark the volume of the gas is back to its original value and its pressure has been increased to a final value where the difference in liquid levels is $h_3 > h_2$. This reflects the fact that we are changing the volume $V$ and the pressure $p$ of the gas at the same time, in such a way that $pV=\text$. Moving the tube on the right changes both the level on the left and also the difference in the liquid levels. We keep adjusting the height of the tube on the right until the liquid on the left is back at the reference mark. The pressure in the gas has increased so the difference in manometer liquid levels increases further to $h_2 > h_1$. The pressure exerted on the gas by the manometer liquid on the left increases, compressing the gas again, moving the liquid level up on the left. This increases the difference in liquid levels even further. Suppose we raise the tube on the right a little.
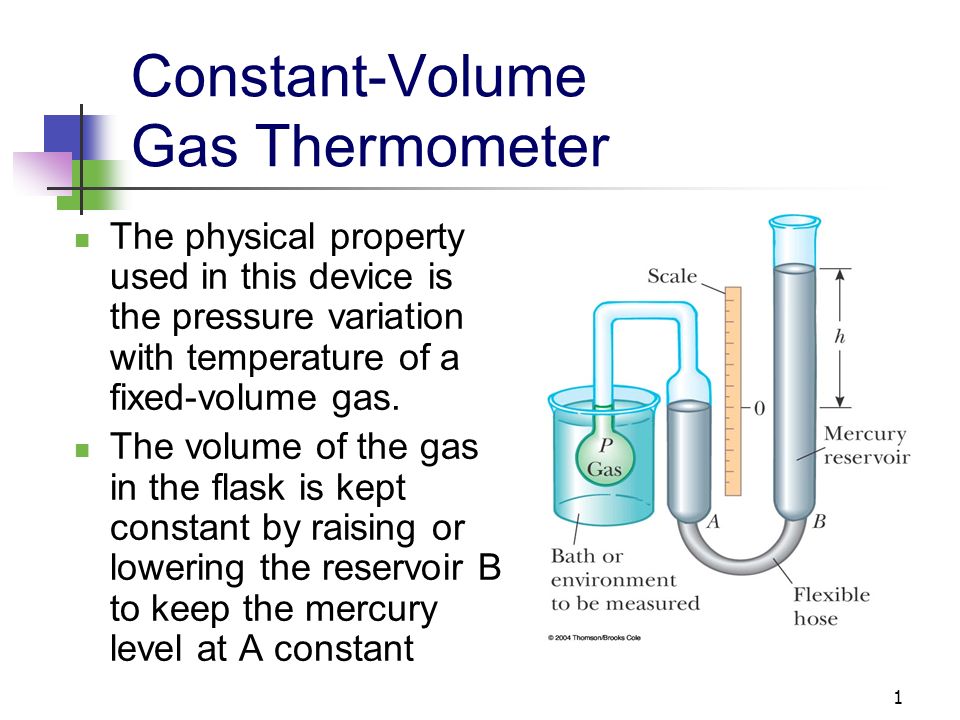
(Imagine cutting out 1cm of flexible tubing on the left and inserting it on the right instead.) The extra liquid comes from the flexible tube, the bottom of which moves up by half as much as the tube on the right. This brings more liquid on the right above the level on the left. To do this the tube on the right is raised up. In order to make a constant volume measurement the liquid level on the left must be raised back to the reference mark. The difference $h$ in liquid levels in the manometer increases from $h$ to $h_1 > h$ because the gas pressure has increased. The manometer level on the left dips below the reference mark, while that on the right rises higher. This increases both the volume $V$ and pressure $p$ of the gas. Suppose that from the diagram the temperature of the gas is increased.


 0 kommentar(er)
0 kommentar(er)
